
When stem cells can’t roll on a bumpy road, muscles break down
Key Takeaways
- Stem cells Cells that have the ability to differentiate into multiple types of cells and make an unlimited number of copies of themselves. Stem cells Cells that have the ability to differentiate into multiple types of cells and make an unlimited number of copies of themselves. travel along a collagen network to reach damaged muscle tissue and heal it.
- In Duchenne muscular dystrophy, stiff, scarred collagen prevents stem cells from reaching their target.
- A protein called sarcospan lessens this scarring and allows stem cells to do their job more successfully, pointing toward potential new treatments for the disorder.
Muscles that ache after a hard workout usually don’t hurt for long, thanks to stem cells that rush to the injured site along “collagen highways” within the muscle and repair the damaged tissue. But if the cells can’t reach their destination, the damaged tissue can’t regenerate. Over time, it breaks down completely and ceases to function.
In a study recently published in npj Regenerative Medicine, a group of researchers led by biochemists at UCLA show for the first time that scarring to the collagen framework that carries these healing cells causes muscles to gradually stop working in Duchenne muscular dystrophy. The discovery in mice illuminates one reason stem cell therapy has not been effective for the disorder: The cells simply can’t get where they’re needed most.
Duchenne muscular dystrophy is the most common — and one of the most severe — hereditary muscular dystrophies. The muscle-wasting disease, which usually affects boys, begins in childhood and inevitably ends in death as the muscles that power the heart, lungs and other vital organs fail. It is caused by a mutation in the gene for the dystrophin protein, which regulates the organization of muscle cells. In healthy people, dystrophin helps bundles of muscle cells called myofibers attach to the collagen framework — the extracellular matrix that gives muscles their shape, holds them together and provides the “highway” for stem cells to repair and regenerate damaged tissue.
Rachelle Crosbie, a UCLA professor of integrative biology and physiology who is looking for ways to treat Duchenne muscular dystrophy, suspected that the dysfunction caused by this mutation led to scarring and stiffening of the extracellular matrix, a process known as fibrosis.
Crosbie and Kristen Stearns-Reider, a postdoctoral fellow in Crosbie’s laboratory, designed a unique experiment to find out. Using facilities at UCLA’s California NanoSystems Institute, they devised a process to “wash” all the cells off the collagen extracellular matrix in healthy mice and those with Duchenne muscular dystrophy.
Under a microscope, the two cell-free matrices, which Crosbie calls “myoscaffolds,” appeared very different: The healthy one looked like delicate lace, while the Duchenne one looked more like a dense sponge.
Next, the researchers seeded each myoscaffold with stem cells and watched as the cells tried to grow muscle tissue. Muscle stem cells grew on the myoscaffolds exactly as they would in healthy and diseased muscle: In the healthy, lacy myoscaffold, cells migrated along the smooth threads and deposited themselves in evenly spaced holes. However, the bumpy, thickened surfaces of the Duchenne myoscaffold made travel difficult and threw up roadblocks that caused the cells to pile up in clumps; the cells were stressed an unable to progress efficiently.
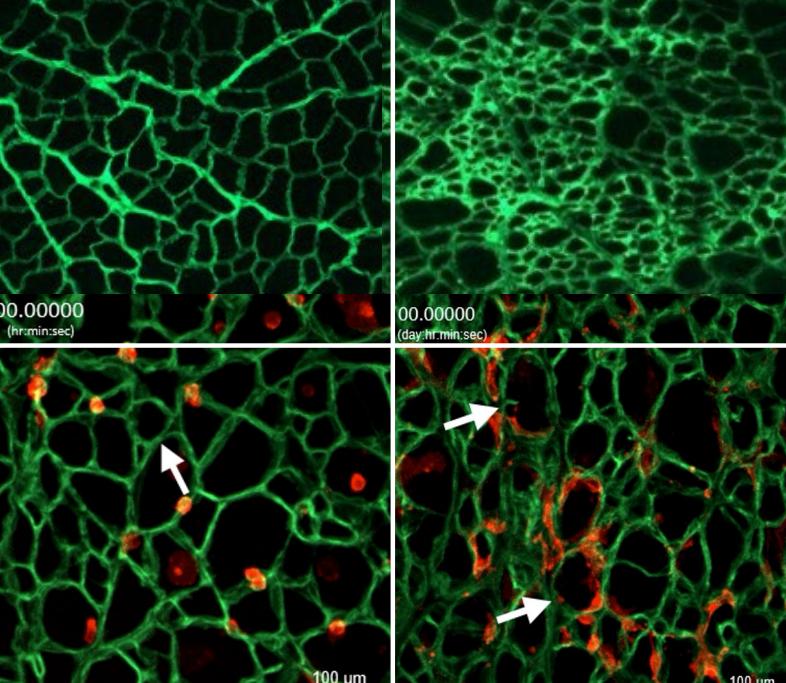
“Like suburban commuters, resident stem cells live on outskirts of the muscle fiber and travel along the muscle fiber to damaged areas and regenerate muscle. The extracellular matrix is the highway they use.” Crosbie said. “It’s like the difference between driving to work on a regular day versus the day a landslide fell on the freeway.”
This is the first time scientists have imaged living cells in a fibrotic myoscaffold, revealing specifically how fibrosis disrupts cell behavior, Crosbie said.
The thin, supple threads of the healthy scaffold also yielded slightly as stem cells attached to them, a deformation critical to the successful development of muscle tissue. The stem cells were unable to deform the thick, stiff fibers of the Duchenne scaffold. Tissue grown on the Duchenne scaffold showed large clumps of myofibers interspersed with even larger clumps of collagen instead of the evenly distributed myofibers seen in the healthy sample.
Protein sarcospan offers a potential way forward
The research team then tested cell behavior on a Duchenne myoscaffold that was created using a therapeutic protein called sarcospan, which is known to stabilize the extracellular matrix. Stem cell function improved once sarcospan had minimized the formation of fibrotic scars.
“The results made it really clear why stem cell therapies have proven challenging for Duchenne muscular dystrophy,” Crosbie said. “Finding ways to prevent or reduce scarring on the extracellular matrix could make them more effective.”
These myoscaffolds offer several broad possibilities for studying stem cell–extracellular matrix interactions, stem cell niche formation, the microenvironments that influence stem cell behavior, muscle maturation and disease modeling, said study co-authors Michael Hicks, a UCLA postdoctoral fellow, and April Pyle, a UCLA professor of microbiology, immunology, and molecular genetics.
Crosbie also noted that because the new method requires only very small samples, these studies could potentially be extended to include individual patients, using tissue from a muscle biopsy to study treatments before they are administered and identifying ones more likely to be effective.
