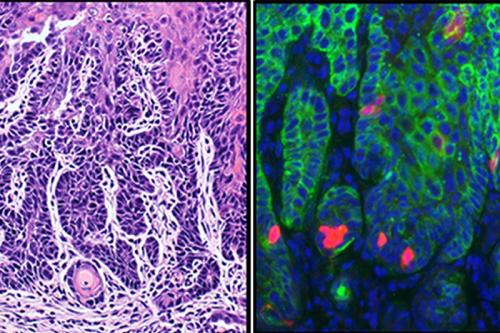
Targeting cancer stem cells improves treatment effectiveness and prevents metastasis
Targeting cancer stem cells may be a more effective way to overcome cancer resistance and prevent the spread of squamous cell carcinoma — the most common head and neck cancer and the second-most common skin cancer, according to a new study by cancer researchers at the UCLA School of Dentistry.
Head and neck squamous cell carcinoma is a highly invasive form of cancer and frequently spreads to the cervical lymph nodes. Currently, cisplatin is the standard therapeutic drug used for people with HNSCC. Yet, more than 50 percent of people who take cisplatin demonstrate resistance to the drug, and they experience a recurrence of the cancer. The five-year survival rates remain sorely low and researchers still don’t understand the underlying mechanisms behind head and neck squamous carcinoma. Therefore, said UCLA cancer biologist Dr. Cun-Yu Wang, who led the study, there’s an urgent need to understand why people with this type of cancer are resistant to therapy and to develop new approaches for treating it.
Wang’s research is published online today in the peer-reviewed journal Cell Stem Cell.
Cancer stem cells are known to be responsible for tumor formation and development; they also self-renew and tend to be unresponsive to cancer therapy. These cells have been found in head and neck squamous cell carcinoma. Given the unique challenges that cancer stem cells pose for oncologists, it remains unclear what the optimal therapeutic strategy is for treating HNSCC.
To address this, Wang, who holds the Dr. No-Hee Park Endowed Chair in Dentistry at UCLA and holds a joint appointment in the UCLA Department of Bioengineering, and his research team first developed a mouse model of head and neck squamous cell carcinoma that allowed them to identity the rare cancer stem cells present in HNSCC using in vivo lineage tracing, a method to identify all progeny of a single cell in tissues.
The researchers found that the cancer stem cells expressed the stem cell protein Bmi1 and had increased activator protein-1, known as AP-1, a transcription factor that controls the expression of multiple cancer-associated genes. Based on these new findings, the UCLA team developed and compared different therapeutic strategies for treating head and neck squamous cell carcinoma. They found that a combination of targeting cancer stem cells and killing the tumor mass, consisting of high proliferating cells, with chemotherapy drugs resulted in better outcomes.
The team further discovered that cancer stem cells were not only responsible for squamous cell carcinoma development, but that they also cause cervical lymph node metastasis.
“This study shows that for the first time, targeting the proliferating tumor mass and dormant cancer stem cells with combination therapy effectively inhibited tumor growth and prevented metastasis compared to monotherapy in mice,” said Wang, who is a member of the UCLA Jonsson Comprehensive Cancer Center and of the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research at UCLA. “Our discovery could be applied to other solid tumors such as breast and colon cancer, which also frequently metastasizes to lymph nodes or distant organs.”
“With this new and exciting study, Dr. Wang and his team have provided the building blocks for understanding the cellular and genetic mechanisms behind squamous cell carcinoma,” said Dr. Paul Krebsbach, dean of the UCLA School of Dentistry. “The work has important translational values. Small molecule inhibitors for cancer stem cells in this study are available or being utilized in clinical trials for other diseases. It will be interesting to conduct a clinical trial to test these inhibitors for head and neck squamous cell carcinoma.”
Additional authors of the study include Demeng Cheng, first author and postdoctoral scholar in Wang’s lab; Mansi Wu, Yang Li, Dr. Insoon Chang, Yuan Quan, Mari Salvo, Peng Deng, Dr. Bo Yu, Yongxin Yu, Jiaqiang Dong, John M. Szymanski, Sivakumar Ramadoss and Jiong Li who are all from the laboratory of molecular signaling in the division of oral biology and medicine at the UCLA School of Dentistry.
This work was supported in part by the National Institute of Dental and Craniofacial Research grants R37DE13848, R01DE15964 and R01DE043110.